The Difference and Similarity Between Osmosis and Diffusion
Are diffusion and osmosis processes related? The first similarity between the two is they involve the movement of molecules from one state of concentration to the other. The difference between osmosis and diffusion is reflected by how these particles move from one concentration point to the other. In definition, diffusion occurs when particles move from an area of high concentration to an area of low concentration. On the other hand, osmosis occurs when solvent particles move from a dilute area (low concentration) to an area of high concentration through a semipermeable membrane.
Differences Between Osmosis and Diffusion
Although both osmosis and diffusion involve particle movements, there are some of the notable differences between them.

- Meaning
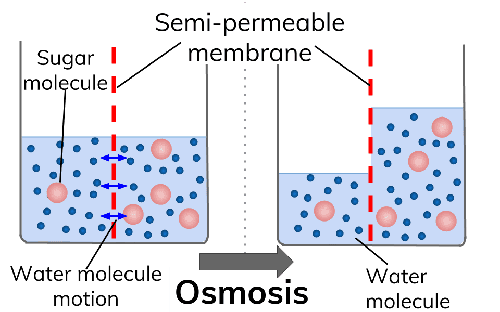
If you consider definition as a way to understand the differences between osmosis and diffusion, it is fairly simple to differentiate between the two. Osmosis is defined as the movement of solvents (liquids) from an area of high solvent concentration to a region of low solvent concentration but through a semipermeable membrane. For example, it is osmosis when water moves from the soil where it's concentered into roots where it's less concentered through a semipermeable root membrane.
On the contrary, diffusion entails different molecules (gas, solid or liquid) movements from an area where molecules are more concentrated to where they are lowly concentrated. Note: the movement must not be through a semipermeable membrane like it is in osmosis.
- Semi-permeable membrane
Considering the passage through which the molecule or solvents move through, you can define osmosis and diffusion. Osmosis happens only through a semipermeable membrane while diffusion happens directly and does not need a semipermeable membrane for it to occur.
- Medium
One main difference between osmosis and diffusion is the medium through which the two happen. Osmosis only happens through liquid mediums that are separated by a semipermeable membrane. Whereas diffusion can happen anywhere and does not require a specific medium. Diffusion can happen through liquid, solids, and gases.
- Molecular diffusion
First, understand there are two types of molecules. There are solvent molecules which are mainly liquids in which solutes (soluble particles) dissolve. With that said, osmosis is the movement of solvents to dilute another concentrated solvent. But diffusion is the movement of solutes, which might be gases, solids or liquids, to less concentrated areas from highly concentrated areas.
- Rate of process
If you consider diffusion and osmosis rates as the defining factors, you will get one major difference between osmosis and diffusion. The main difference is that osmosis is a slow process, whereas diffusion is a faster process. This can be attributed to factors like the necessity of a semi-permeable membrane. Although that membrane allows solvent molecules to pass through, it does hinder their fast movement to some extent. Secondly, when considering the fact that diffusion moves from an area of high concentration to an area of low concentration, this implies a high rate. On osmosis, the opposite happens; it is the movement of solutes from an area of low concentration to an area of high solute concentration which results in the slowness of osmosis rate.
- Free-energy
Depending on free-energy fact, osmosis depends on one single solvent which is water. Osmosis seeks to equalize solvent concentrations by reducing free energy from one part and adding it to the other although an equilibrium (equalization) point never eventuated. Further, diffusion entails the movement of molecules from an area of high free energy to where there is lower energy to achieve an equalized energy concentration.
- Importance
To animals, osmosis helps in maintaining cellular water levels, nutrient transportation, and movement of molecules from one cell to the other; commonly known as cell-cell diffusion. Diffusion in animals as well helps in their energy creation during respiration as gases get exchanged.
Although there are differences between osmosis and diffusion, both are helpful to plants. Osmosis helps plants by sustaining water absorption that leads to plant cell turgidity. Cell turgidity results in mechanical plant support (steady plant standing). Osmosis also prevents plants from losing excess water. Lastly, diffusion does help plants in gaseous exchange thus supporting photosynthesis and transpiration.
Similarity Between Osmosis and Diffusion
Although osmosis and diffusion are somehow different, they have one main similarity. They are all passive transport processes. Being passive means both require no extra energy to take place. This is because they all revolve around one thing: the movement of particles from a high concentration to those areas of low concentration. Thus, these particle movements are free from energy need.
In conclusion, both osmosis and diffusion are significant happenings in the science field. Both are physical processes that are significant to human and the general environment. They are natural processes of molecules intermingling which helps in maintaining different plant body equilibriums.
YOU MAY LIKE
-
Which Are the States of Matter and Change of States
-
Major Advantages and Disadvantages of the Internet
-
20 Best Party Songs of All Time
-
9 Best Games to Play With Friends
-
9 Steps on How to Write a Lab Report
-
Wondering Why You Cannot See His/Her Posts on Instagram?
-
Detailed Steps for Converting Temperatures
-
Nine Most Notable Red and Black Bugs You Might Come Across
-
What Should You Do if Someone Drinks Bleach?
-
How and Where to Sell Your Beanie Babies
