3 Types of Elements on the Periodic Table and Their Properties
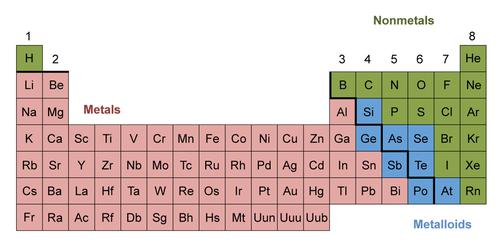
Depending on the properties of metals nonmetals and metalloids, a table of illustration known as the periodic table is drawn. A periodic table is vital when one is trying to understand elements regarding their properties and reaction levels. A periodic table has stairs like steps that help identify the element by groups. For easy classification of elements, there are two main classes: metals and non-metals. But, elements that exhibit both metallic-like and nonmetallic properties make another class of semimetals or metalloids. On the periodic table, those elements on the far left are metals followed by metalloids and those on the far right are nonmetals. Hydrogen (H), which at standard pressure and temperatures behaves like a nonmetal, is the first element on a periodic table.
Properties of Metals, Nonmetals, and Metalloids
By understanding various properties of metals nonmetals and metalloids, you will better be able to distinguish different states of matter. Below are explanations on physical and chemical properties of various periodic table elements.
1. Metals
When you look at the periodic table, all the elements starting from Boron (B) of atomic number 5 to Polonium (Po) of atomic number 84, all those are metals except Antimony (Sb), Germanium (Ge) and five other elements.
Physical properties
-
State: Metals are found in solid states when at room temperature, although mercury is a metal in liquid form.
-
Luster: When metals are polished, for example silver, copper or gold, they can reflect light that falls on their surface.
-
Malleable: When metals get hammered, they do withstand hammering although they get spread out to form thin sheets of whatever size that is hammered.
-
Good conductors: Metals have free electrons which are responsible for electrical conduction. Copper and silver are the best conductors while lead is the poorest metal conductor.
-
Density: Metals' density is very high which results in their heaviness.
-
Lastly, metals have high melting points.
Chemical properties
-
When metals are oxidized (undergo a chemical reaction), they lose their electrons when bonding. This is because they have low ionization energy and usually do not accept electrons after a chemical reaction.
-
Metals are easily affected by corrosion.
2. Nonmetals
When studying properties of metals nonmetals and metalloids, elements found on the far right of a periodic table are known as nonmetals. Below are their properties.
Physical properties
-
State: At room temperatures, nonmetals are mainly found in two primary forms: gaseous form, for example oxygen, and solid form, like carbon. The exception is bromine, the only nonmetal that exists as a liquid.
-
Non-malleable and non-ductile: All nonmetals are very brittle. They cannot get pounded to make sheets or rolled into any sized wires respectively.
-
Poor conductor: Nonmetals lack free ions. For that reason, nonmetals are poor conductors of electricity as well as heat.
-
Luster: It is impossible to polish gas or a nonmetal solid. These do not and cannot reflect any light that falls on their surfaces.
-
Melting points: Particle, atoms or molecules that form nonmetals like gas and some solids are less compacted. Thus, non-metal boiling points are never high. Generally, they are lower compared to those of metals.
-
Diatoms: Seven types of nonmetals at standard condition exist as diatomic molecules. They include H2, N2, O2, F2, and CL2 all gases, Br2 which is a liquid and lastly I2 which is a solid.

Chemical properties
-
Nonmetals do exhibit an electronegative property in which they tend to gain or share electrons as they react and bond with other atoms. When they react with metals, nonmetals gain electrons, for example from metals.
-
Nonmetals react with each other to form compounds joined through covalent bonding. Mostly, they form neutral oxides or acids that can dissolve in water to form acids.
3. Metalloids
Within periodic table metals and nonmetals, you get metalloids. They are elements with properties from both metals and nonmetals. Metalloids are Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium and Astatine. Metalloid properties are as follows:
Physical properties
Metalloid exhibit metal like physical properties:
-
Semiconductors: When metalloids are under the right condition, they can conduct some electricity.
-
State: When at room temperatures, metalloids are solids.
-
Some metalloids like silicon do appear lustrous although they are not ductile or malleable.
Chemical properties Although metalloids are mixed with periodic table metals, they exhibit metallic properties on their physicals properties but nonmetallic properties on chemical properties.
-
When bonding with other elements, metalloids lose and gain electrons respectively. When metalloids found on the right react, they gain electrons. For those that are on the left, when they bond they end up losing electrons.
-
Metalloids oxidation ranges between +5 and -2.
Study of the periodic table helps people understand the different types of elements available. It is through metal study that various manufacturing industries thrive. Human beings depend on chemical reactions to understand metals, metalloids, and nonmetals. A little concentration will help you understand and be able to locate atoms on the periodic table.
YOU MAY LIKE
-
Disadvantages and Advantages to Keep Animals in Zoos
-
5 Steps to Draw Lewis Structures
-
Do You Know Why People Don’t Read Books?
-
Most Interesting Challenges to Do With Your Friends
-
What Are the 3 Parts of a Nucleotide and Nucleotide Examples
-
Tips on How to Write a “Thank You” E-mail
-
11 Amazing Chemical Reactions in Everyday Life!
-
The Significance of Knowing Your Strengths and Weaknesses
-
Different Truth or Dare Questions for Adults
-
DNA VS RNA: A Comparative Study
